Citric Acid Ionic or Covalent
Hydrocyanic acid 27026 gmol. 160 ml NaSiO 3 and 5 ml ethanol were dripped into above dispersion of RGO-CNTs under room temperature.

Formation Of An Ionic Bond Between The Precationized Cotton And Citric Download Scientific Diagram
Choose all that apply.
. In acid-base reactions this usually means dissolving in water. Which of the following statements about enzymes are true. C 2 H 3 NaO 2.
Usually the acidic substances are identified with their sour taste. A process that takes place when a covalent compound reacts with water to form new ions OR Breaking up of a molecule into charged components ions. Ionic complementary polypeptides can self-assemble into stable β-sheet nanofibers through electrostatic interactions.
The pK a value is directly proportional to the standard Gibbs free energy change for the reaction. On the basis of the DFT calculations table S1 P-T generally has quadruple hydrogen bonds with interaction energy of 6836 kcalmol and the averaged interaction energy 1709 kcalmol is within the range of hydrogen bond of. How are elements numbered 58 to 71 in the periodic table called.
The acid definition is given as any hydrogen that contains a substance capable of donating a proton a hydrogen ion to the other substance. A culture of the fungus aspergillus niger is used industrially in the manufacture of citric acid and other organic species. While the ATP yield of the citric acid cycle is modest the generation of coenzymes NADH and FADH 2 is critical for ATP production in the final stage of cellular respiration oxidative phosphorylation.
C 6 H 8 O 7. An acid is a substance which can donate hydrogen ions and a base is a substance which can donate hydroxide ions in an. Lemon juice citric acid vinegar acetic acid stomach acid and soda pop carbonic acid.
Ionic complementary polypeptides are chains of amino acids which can be synthesized using charged amino acid residues such as positively charged lysine K and arginine R and negatively charged glutamic acid E and aspartic acid D. C 7 H 6 O 3. The value of the pK a changes with temperature and can be understood qualitatively based on Le Châteliers principle.
There are many common acid substances. Group of answer choices. Ionic Video Take Quiz Lesson 5 - Chemical Bonds III.
C Ionic compounds conduct electricity when dissolved in water or when melted because they contain ions charged particles. What are acids base and salt. Ionic Chemical Bonds II.
These coenzymes act as electron carriers and donate. Cells of the fungus have an ultimate analysis of mathrmCH_179 mathrmN_02 mathrmO_05 and the heat of formation of this species is necessary to approximate the heat duty for the bioreactor in which citric acid is to be produced. Hydrogen ions are the basis for all acids and one definition of an acid is that it is a hydrogen ion donor.
A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F Enzymes lower. B Ionic compounds are soluble in water but covalent compounds are insoluble in water. Mg 3 PO 4 2.
Polar Covalent Chemical Bonds III. Lesson 4 - Chemical Bonds II. Basically an acid is a molecule that can donate an H ion and also can remain energetically.
Fe 2 O 3. Covalent compounds have weak force of attraction between their molecules so they are usually liquids or gases. The citric acid cycle also regenerates oxaloacetate the molecule that starts the cycle.
A base is an ion or molecule that is able to accept a hydrogen ion from an acid. After that the mixture was heated at 30 C under string for 10 h and the dispersion of non-covalent modified RGO-CNTs was obtained. The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction.
What is considered as the general purpose oldest type and widely used cast iron. Acids are typically ionic with a positive hydrogen ion attached to a negative anion. Ionic bonding.
Compounds such as the citric acid in lemon juice the ethanoic acid in vinegar or a typical laboratory acid like hydrochloric acid all give their hydrogen ions away in chemical reactions known as acid-base reactions. After that the mixture was heated under 85 C and 100 ml citric acid was added. When all atom molecule are the.
A process that take place when an ionic compound dissolves in water allowing the ions in the compound to separate. The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction.
Chemical Structure Of Citric Acid And Maletic Acid Download Scientific Diagram
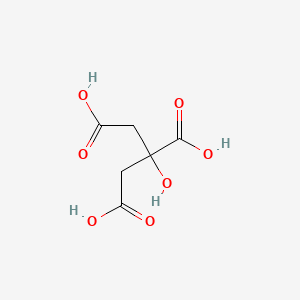

0 Response to "Citric Acid Ionic or Covalent"
Post a Comment